Accumulation of oxidised extracellular matrix proteins in tissues exposed to low grade inflammation.
Immune system cells are a major source of oxidants. Leukocyte activation generates O2-. and H2O2, and results in myeloperoxidase (MPO) release. MPO uses H2O2 and Cl- to generate the powerful oxidant HOCl. This mediates pathogen killing, but misplaced formation is linked with extracellular matrix damage (via MPO binding) and human disease.
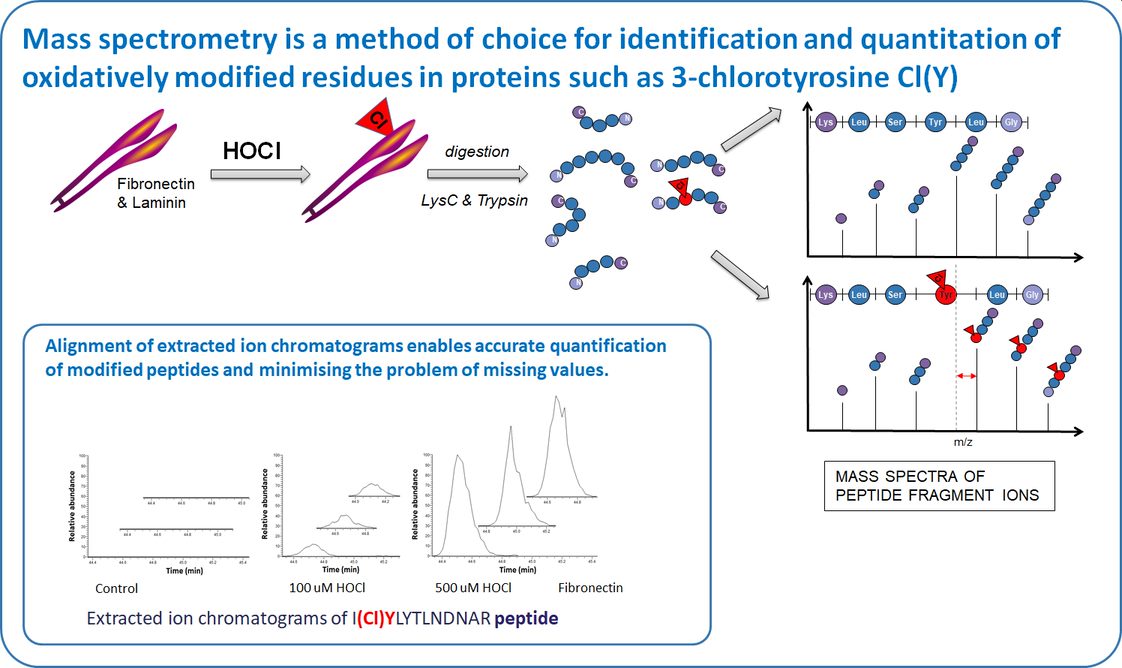
Our study focuses on quantification of chlorination (3-chloroTyr) and oxidation of human extracellular matrix proteins: fibronectin, laminin and tropoelastin induced by HOCl and an enzymatic MPO system, and its consequences for human coronary artery smooth muscle cell adhesion, proliferation, migration and gene expression.
We use mass spectrometry for identification and quantification of protein modifications because of its sensitivity and specificity. We have optimised sample preparation and analysis protocol to detect and quantify 3-chloroTyr and other readily-oxidized residues such as Met, His, Cys, and Trp. Using label-free quantification at a peptide level, in shotgun MS of single proteins, chlorination can be detected down to a relative site occupancy of ~0.15%.
We observe that 3-ChloroTyr and other amino acids oxidations are detected at multiple sites on fibronectin, laminin and tropoelastin, including within functionally-important heparin-, cell- and collagen-binding domains, in a dose-dependent manner. Damage is localized to specific residues and occurs to markedly different extents. For example, heparin-binding and cell adhesion to HOCl-modified fibronectin is decreased in a dose-dependent manner. Cell proliferation on HOCl-damaged fibronectin is modulated, and migration attenuated. Expression of matrix proteins and matrix metalloproteinases in cells exposed to modified fibronectin is significantly altered.
Related publications
- Mariotti, M.; Rogowska-Wrzesinska, A.; Hägglund, P.; Davies, M. J. (2021): Cross-linking and modification of fibronectin by peroxynitrous acid: Mapping and quantification of damage provides a new model for domain interactions. J Biol Chem 296, 100360. http://dx.doi.org/10.1016/j.jbc.2021.100360.
- Zhao, Z.; Engholm-Keller, K.; Poojary, M. M.; Boelt, S. G.; Rogowska-Wrzesinska, A.; Skibsted, L. H.; Davies, M. J.; Lund, M. N. (2020): Generation of Aggregates of α-Lactalbumin by UV-B Light Exposure. J Agric Food Chem 68, 24, 6701-6714. http://dx.doi.org/10.1021/acs.jafc.0c00757.
- Carroll, L.; Jiang, S.; Irnstorfer, J.; Beneyto, S.; Ignasiak, M. T.; Rasmussen, L. M.; Rogowska-Wrzesinska, A.; Davies, M. J. (2020): Oxidant-induced glutathionylation at protein disulfide bonds. Free Radic Biol Med 160, 513-525. http://dx.doi.org/10.1016/j.freeradbiomed.2020.08.018.
- Nybo, T.; Dieterich, S.; Gamon, L. F.; Chuang, C. Y.; Hammer, A.; Hoefler, G.; Malle, E.; Rogowska-Wrzesinska, A.; Davies, M. J. (2019): Chlorination and oxidation of the extracellular matrix protein laminin and basement membrane extracts by hypochlorous acid and myeloperoxidase. Redox Biol 20, 496-513. http://dx.doi.org/10.1016/j.redox.2018.10.022.
- Nybo, T.; Davies, M. J.; Rogowska-Wrzesinska, A. (2019): Analysis of protein chlorination by mass spectrometry. Redox Biol 26, 101236. http://dx.doi.org/10.1016/j.redox.2019.101236.
- Lorentzen, L. G.; Chuang, C. Y.; Rogowska-Wrzesinska, A.; Davies, M. J. (2019): Identification and quantification of sites of nitration and oxidation in the key matrix protein laminin and the structural consequences of these modifications. Redox Biol 24, 101226. http://dx.doi.org/10.1016/j.redox.2019.101226.
- Nybo, T.; Cai, H.; Chuang, C. Y.; Gamon, L. F.; Rogowska-Wrzesinska, A.; Davies, M. J. (2018): Chlorination and oxidation of human plasma fibronectin by myeloperoxidase-derived oxidants, and its consequences for smooth muscle cell function. Redox Biol 19, 388-400. http://dx.doi.org/10.1016/j.redox.2018.09.005.