
New project will study molecules to understand why you are exactly you
Throughout your life, various influences can turn on and off many of your genes, creating the variations that make you uniquely you. Chemistry professor Jasmin Mecinovic wants to delve into the molecular world to understand these processes.
Most people know that DNA, our genetic material, plays a crucial role in our development, appearance, and susceptibility to diseases. This DNA exists in every one of our cells and remains unchanged; when a cell divides to renew itself, it makes an exact copy of its DNA. But if every cell starts out with the same DNA, why do all cells not develop the same way?
This is what science calls a very good question, and in recent years, many scientists have turned their focus to epigenetics; that is, how external factors can, for shorter or longer periods, alter the expression of otherwise unchangeable genes within a cell.
Such factors can be what we eat, how we exercise, or how we age. Some researchers also believe that epigenetic changes can be passed from a parent to the unborn child, essentially coding the child to adapt to the environment the parent lives in. Some researchers further argue that psychological influences, such as stress and trauma in the parents, can lead to epigenetic changes in an unborn child, for example elevated levels of stress hormones.
Epigenetic changes are not permanent and can be reversed.
About the project
Jasmin Mecinovic has received 13,209,000 DKK from the Novo Nordisk Foundation for the project "Understanding the Chemical Language of Linker Histones."
Molecules form the basis of all life
- All life is molecular at its core. So, the idea that organisms respond to influences at the molecular level is logical. I am a chemist, and I am interested in what happens at the molecular level when a specific epigenetic modification affects a gene, says Jasmin Mecinovic.
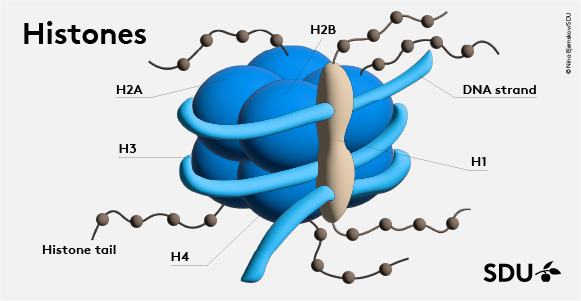
Epigenetic influences - whether they are intended by nature or triggered by environmental factors - initiate chemical changes in certain structures surrounding DNA inside every cell nucleus. These protein structures are called histones, and their primary function is to provide a framework around which the two-meter-long DNA strands can coil.
But they also metaphorically have a tail that extends outside of the DNA. Enzymes can attach or remove small chemical groups to this tail. When enzymes do this, it sends a signal through the histone into the cell nucleus, that certain genes inside should be turned on or off.
A genome is the entire genetic material of an organism and contains a tremendous amount of information - most people have likely heard of the bases A, C, G, and T, which can be connected in countless ways between the two DNA strands. Each connection leads to a unique gene, which, in turn, determines the function of the cell in an organism. In simple terms, the composition of these bases is responsible for traits such as hair color, handedness, or the development of extra toes.
- A human genome contains at least 20,000 genes, so let’s compare it to a book with 20,000 pages: It contains a lot of information, but not everything is in play at the same time. Let's pretend that we both have a copy of that book. I open it at page 10, and you open it at page 100. We get two different pieces of information from the same source. That's how it works with genes; not everything is accessible all the time, only specific parts are activated. And this activation can be triggered by proteins that attach, remove or recognize small chemical tags on histones, explains Jasmin Mecinovic.
How and where enzymes attach chemical groups on the histones, and how such changes control the gene expression, is a huge puzzle that researchers have only had the opportunity to explore in the last 30 years. The changes are so small that extremely advanced chemical tools are needed to peer into the world of histones.
Fluttering around with spaghetti arms
For the same reason, researchers have mostly focused on the four core histones around which DNA strands coil. But there is a fifth histone, called linker H1, which is so unruly that, until very recently, it has been impossible to study using existing techniques.
- Imagine that this histone has spaghetti arms that are constantly fluttering around, making it difficult to take a picture of it; it's like if I wave my hand while you take a picture, it becomes blurry. But this histone exists in humans and in other complex life forms, so it must serve an important function, and that's what I want to investigate, says Jasmin Mecinovic.
One of the core tasks for this new project is to develop chemical methods that can reveal what happens in the epigenetic corners of the unruly H1 histone.
Causing diseases
Like the other histones, H1 also has a tail that extends outside the DNA. Enzymes can also modify it and modify genes in the cell nucleus via the linker histone.
- So, another core task will be to attempt to construct H1 histones that we can specifically modify to look like the natural H1, in order to see what effect it can have on interactions with histones, epigenetic proteins and DNA, explains Jasmin Mecinovic.
Studying how H1 histone interacts with other biomolecules at the molecular level is basic chemical research. However, it is also research related to diseases and medicine, as histones can cause undesirable activations or suppressions of genes, leading to diseases such as certain cancers and autoimmune diseases such as rheumatoid arthritis, lupus, type 1 diabetes, and multiple sclerosis.
Meet the researcher
Jasmin Mecinovic is a professor of chemistry at the Department of Physics, Chemistry, and Pharmacy. After studies at Oxford and Harvard, he joined SDU in 2018. His group uses chemistry to understand biological processes at the molecular level. His research is currently supported by the Novo Nordisk Foundation, Lundbeck Foundation, and the Danish Independent Research Fund.