
Associate professor Soren Werner Karlskov Hansen
Cancer and Inflammation Research
CV, Research Output
Phone +45 6550 3062
Email: shansen@health.sdu.dk
Research Interests
Innate immunity, pattern recognition molecules and the complement system
The reason we are healthy most of the time is due to a part of our immune system referred to as our innate immune system. It deals more or less “silently” with microorganisms, without our noticing and often in absence of any sign of disease. When microorganisms develop into pathogens that colonize our body, the pathogens usually escape the attention or effect of the innate immune system, and we have to rely on our adaptive immune system (T and B cells); with the result of severe inflammation, fever and prolonged disease. A particular important part of the innate immune system is the complement system, which is found in the blood as inactive components (proteins) that activate upon the encountering of microorganisms. Complement activation leads to covalent “tagging” (deposition) of complement products on the microbial surface, and thereby enhanced clearance by phagocytes, attraction of granulocytes and even lysis (direct killing) of the microorganisms. Three distinct activation pathways can activate the complement system: the classical, the alternative and the lectin pathway. We have been involved in the initial discovery of the lectin pathway, which relies on recognition of non-self microbial glycoconjugates (Fig. 1). Recently, we were the pioneers in the discovery of novel pattern recognition molecules that in complex with each other activate the complement system efficiently also via the lectin pathway. These molecules comprise the collectins; collectin kidney 1 (CL-K1) and collectin liver 1 (Cl-L1) and their heteromeric complex, referred to as CL-LK (Fig. 1).
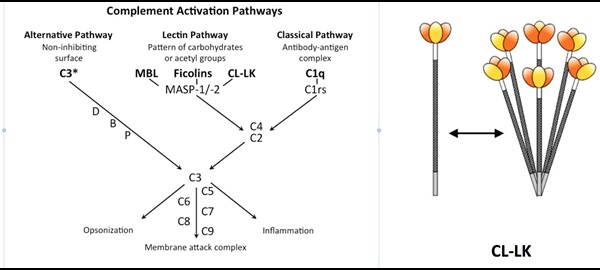
We are currently in different projects investigating the role of CL-LK (CL-K1 and CL-L1) in the development and pathogenesis of infections, autoimmune diseases and thrombosis (interaction with the coagulation system). Our main hypotheses are that complement activation mediated by CL-LK is a crucial factor in these diseases. Low CL-LK levels and lack of complement activation can be associated with infections, whereas high levels and expression of altered self glycoconjugates can be associated with the development of complement-mediated autoimmune diseases and thrombosis.
Therapeutic monoclonal antibodies: personal treatment and response
Therapeutic antibodies are a developing field for treatment of an expanding number of inflammatory diseases including Crohn’s disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis and psoriasis arthritis. However, the treatment is frequently hampered by development of anti-drug antibodies that may lead to anaphylactic reactions and/or inefficient treatment. In order to identify and characterize non-responding patients, we developed assays for addressing quantification of the patient’s own anti-drug antibodies, with emphasis on anti-drug antibodies that neutralize the given therapeutic antibodies. We have taken our starting point in the anti-TNFα antibody (Infliximab / Remicade / Remsima) (Fig. 2) but the setup is applicable to other therapeutic antibodies. We are currently analyzing the benefits of our assays in a cohort of patients with inflammatory bowel diseases. Reliable assessment of immunogenicity and half-life of anti-TNFα and other biologicals could be used to optimize dose regimens and prevent prolonged use of inadequate therapy, with a perspective of economizing the health system’s usage of therapeutic antibodies, which is an increasing burden.

Non-invasive markers for the severity and progression of haematological diseases
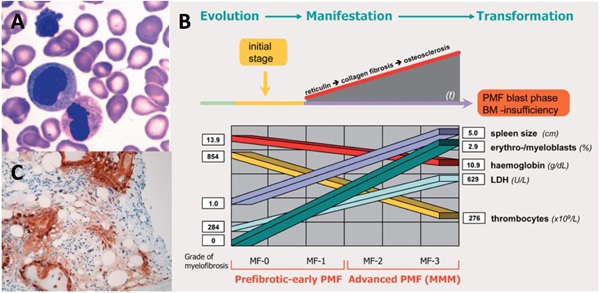
The haematological disease, primary myelofibrosis (PMF) is a chronic myeloproliferative neoplasm that is characterised by bone marrow fibrosis, splenomegaly, constitutional symptoms and anaemia. In other myeloproliferative neoplasms and PMF particular, the closely regulated balance between bone marrow matrix synthesis and degradation is altered. Consequently, neo-angiogenesis, stromal proliferation and extensive bone marrow fibrosis are all inherent features. The treatment strategy for myelofibrosis varies considerably depending upon the phenotype, severity and progression of the disease and requires specialized examination of the patient’s bone marrow by immunohistochemistry. We have found that procollagen type I (PINP), which is synthesised and secreted from fibroblasts and osteoblasts, may be a potential biomarker for PMF severity and progression (Fig. 3). We are currently characterizing the association between PINP blood levels and
disease severity in a cohort of patients with myeloproliferative neoplasms.
Members
- Stud. scient. Nichlas Littau Lilja
- Lab manager / technician, Ph.D, Maiken Lumby Henriksen
- Stud. scient. Nicoline Ostermann Lorenzen
- Stud. scient. Jacqueline Ann Soucy
- Associate professor, Ph.D Søren W. K. Hansen
- Stud. scient. Karen Birkkjær Bjerrum
- Stud. scient. Josephine Baunvig Aagaard
- Stud. polyt Rasmus Skammelsen

Bibliographic statistics
Google Scholar (up to date H-index and more)
Orchid ID: 0000-0002-8186-4630
ResearchGate
Information to new students
As per 2016/2017 we are currently seeking highly motivated students in our research areas of: “Innate immunity, pattern recognition molecules and the complement system” and “Therapeutic monoclonal antibodies – personal treatment and response”
Contact Soren W. K. Hansen pr. mail or by personal contact at the office or lab. at J.B. Winsloews Vej 21, 1st floor.
Acknowledgements
Without external funding we would not be able to do much science. Amongst others, we are grateful and in debt to following foundations:
A. P Møller Fonden
Augustinusfonden
Dagmar Marshalls Fond
Familien Hede Nielsens Fond
Frimodt-Heineke Fonden
Højbjerg fonden