New insights into aggressive breast cancer and potential treatment options
Patients with triple-negative breast cancer may find encouragement in recently published research from Professor Vijay Tiwari from the Department of Molecular Medicine.
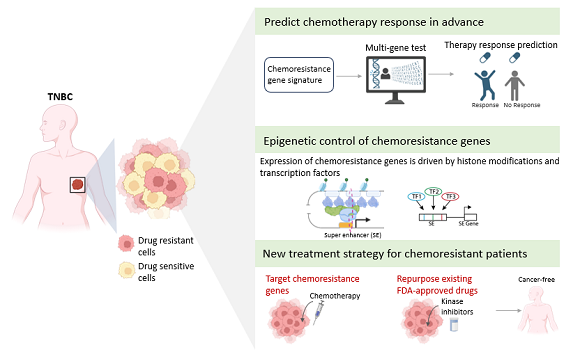
Triple-Negative Breast Cancer (TNBC) is difficult to treat due to its aggressive nature and resistance to chemotherapy. Exciting research published from the University of Southern Denmark sheds light on the mechanisms that drive this resistance and gives hope for better treatment for patients in the future.
Two separate studies, conducted by a team of researchers from the Department of Molecular Medicine, published in the journals Precision Oncology and EMBO Molecular Medicine, have delved into the understanding of resistance to chemotherapy in TNBC and found new ways to segment patients and improve treatment from it.
What is a biomarker?
A biomarker, also known as a biological marker, refers to a quantifiable sign of a biological state or condition.
Biomarkers are frequently assessed and analyzed using blood, urine, or soft tissues to investigate typical biological functions, disease processes, or the body's response to medical treatments.
Professor Vijay Tiwari, leader of the research group, says:
-Our findings have identified specific groups of cells in the tumor that drive resistance to chemotherapy and further decoded the underlying molecular program that confers this behavior including the signaling and survival cues with the tumor niche. Furthermore, genes expressed by these cells offer the best-in-class biomarker to predict chemotherapy response and targets for therapy using existing FDA-approved drugs. It is a truly exciting development with the potential to significantly improve the lives of TNBC patients.
Special resistant cells
In the first study, the researchers uncovered previously unknown types of cells in TNBC tumors. It is precisely these cells that show signs of resistance to chemotherapy. The study further identified genes that confer resistance properties to these cells.
Postdoc Mohammed Inayatullah, lead author of this study, used advanced genomics tools combined with machine learning to make this discovery.
- We have gained an increased understanding of the mechanisms behind drug resistance, and have the potential to uncover robust biomarkers for developing better treatment strategies in difficult-to-treat cancers such as TNBC, he says.
The study also points to potential alternative treatment options for TNBC patients who are resistant to chemotherapy.
→ Find the study via this link
Epigenetic control of resistance
The second study, led by PhD student Ryan Lusby, focused on unraveling the epigenetic mechanisms that drive chemoresistance in TNBC. Using data from patients with TNBC, the authors have elucidated how a specific chemical modification on histone proteins controlled chemoresistance genes.
What is epigenetics and histone proteins?
Epigenetics involves examining how behaviors and environmental factors induce alterations that impact gene functionality.
Unlike genetic modifications, epigenetic alterations are reversible and do not alter the DNA sequence; however, they can influence the interpretation of a DNA sequence by the body.
Histone proteins are vital components that offer structural reinforcement to chromosomes. Within each chromosome lies an extensive DNA molecule that necessitates accommodation within the cell nucleus. This accommodation is facilitated by the DNA winding around clusters of histone proteins, thereby compacting the chromosome's structure.
-By comprehensively mapping this modification in TNBC patients, we found in our study some so-called super enhancers that drive expression of genes crucial for chemotherapy resistance, says Ryan Lusby. Notably, loss of transcription factors occupying these super enhancers overcame resistance.
This study has revealed how targeting genetic and epigenetic mechanisms underlying chemoresistance offers novel avenues for therapy.
→ Find the study via this link
Meet the researcher
Vijay Tiwari. Professor & Head of Genome Biology, Institute for Molecular Medicine, SDU.